
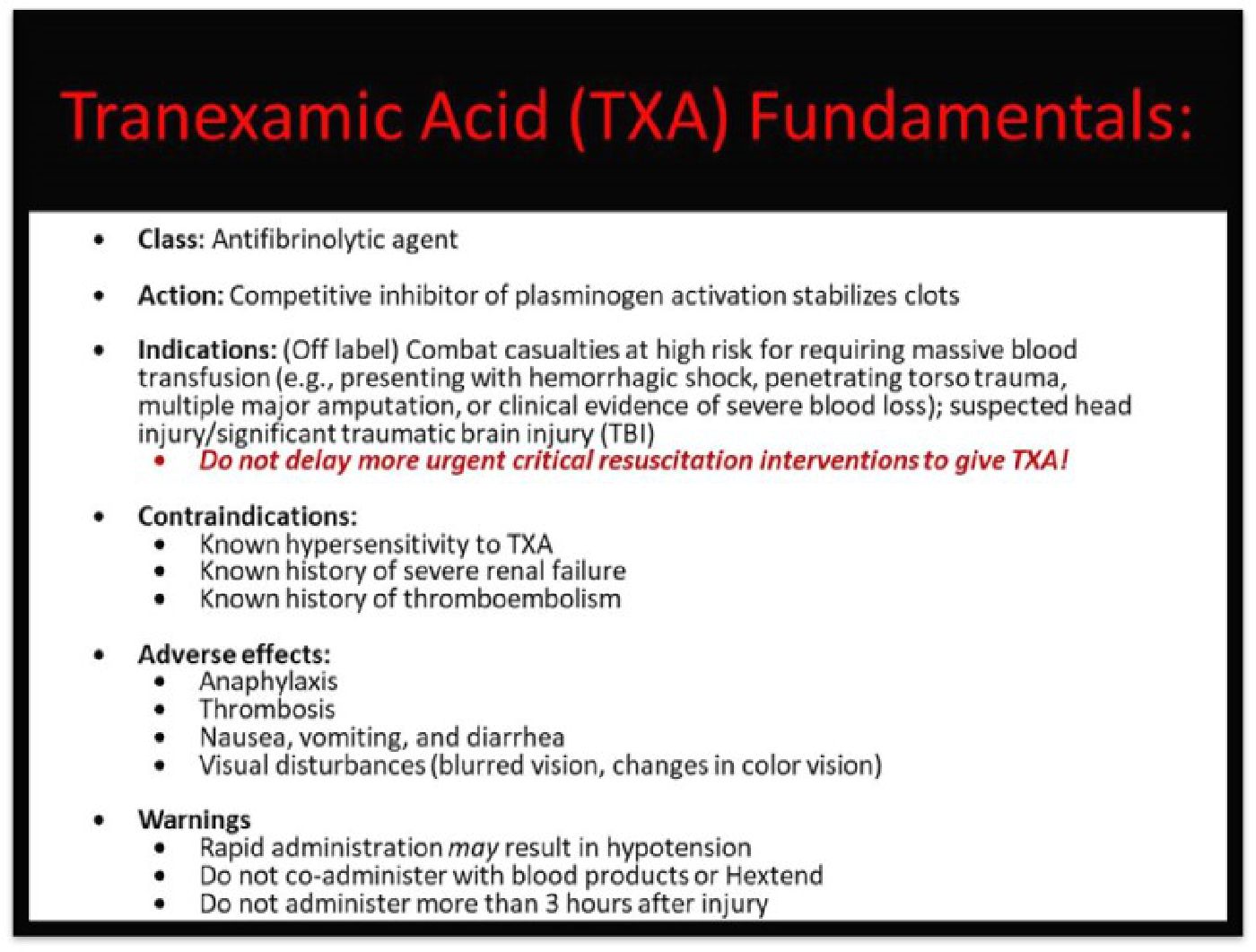
Tranexamic Acid (TXA) is an inexpensive, antifibrinolytic agent shown to reduce morbidity and mortality in patients with significant bleeding. In 2011, the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage-2 (CRASH-2) trial demonstrated TXA's efficacy in improving outcomes in patients with significant blood loss or hemorrhagic shock. Shortly thereafter, the Department of Defense's Committee for Tactical Combat Casualty Care (CoTCCC) adopted the use of TXA in its guidelines. Since 2011, TXA has been used extensively in military and civilian pre-hospital and hospital settings during trauma resuscitation, and used prophylactically during surgery. Based on new evidence-based research, TXA's indications have expanded to include closed head injury, greater emphasis on early administration, and a reexamination of administration and dosage. Our aim is to provide the key evidence for the benefits of administering intravenous TXA to trauma patient with massive hemorrhage, and to provide the rationale for the 2020 update of Tactical Combat Casualty Care (TCCC) guidelines for TXA use.
In 2019, CoTCCC convened a working group to examine proposed changes to TXA use. Drew et al. reviewed 165 studies pertinent to TXA’s use in the operational setting.
Background
At the time of TXA's introduction into the Tactical Combat Casualty Care (TCCC) guidelines, hemorrhage was, and remains, the leading cause of preventable death on the battlefield. In a retrospective analysis of combat casualties in Iraq and Afghanistan, Kelly et al. concluded that hemorrhage contributed to 85% of deaths. Additionally, the authors concluded, 9% of potentially survivable patients died of central nervous system injuries primarily traumatic brain injury (TBI).
The evidence for TXA's efficacy in treating massive blood loss and hemorrhagic shock remains robust, especially when administered promptly and to the appropriate patient. The last decade has seen an abundance of literature that supports TXA's use in trauma and reinforced early administration. The CRASH-2 trial found that timely administration of TXA is essential to confer the maximum benefit.
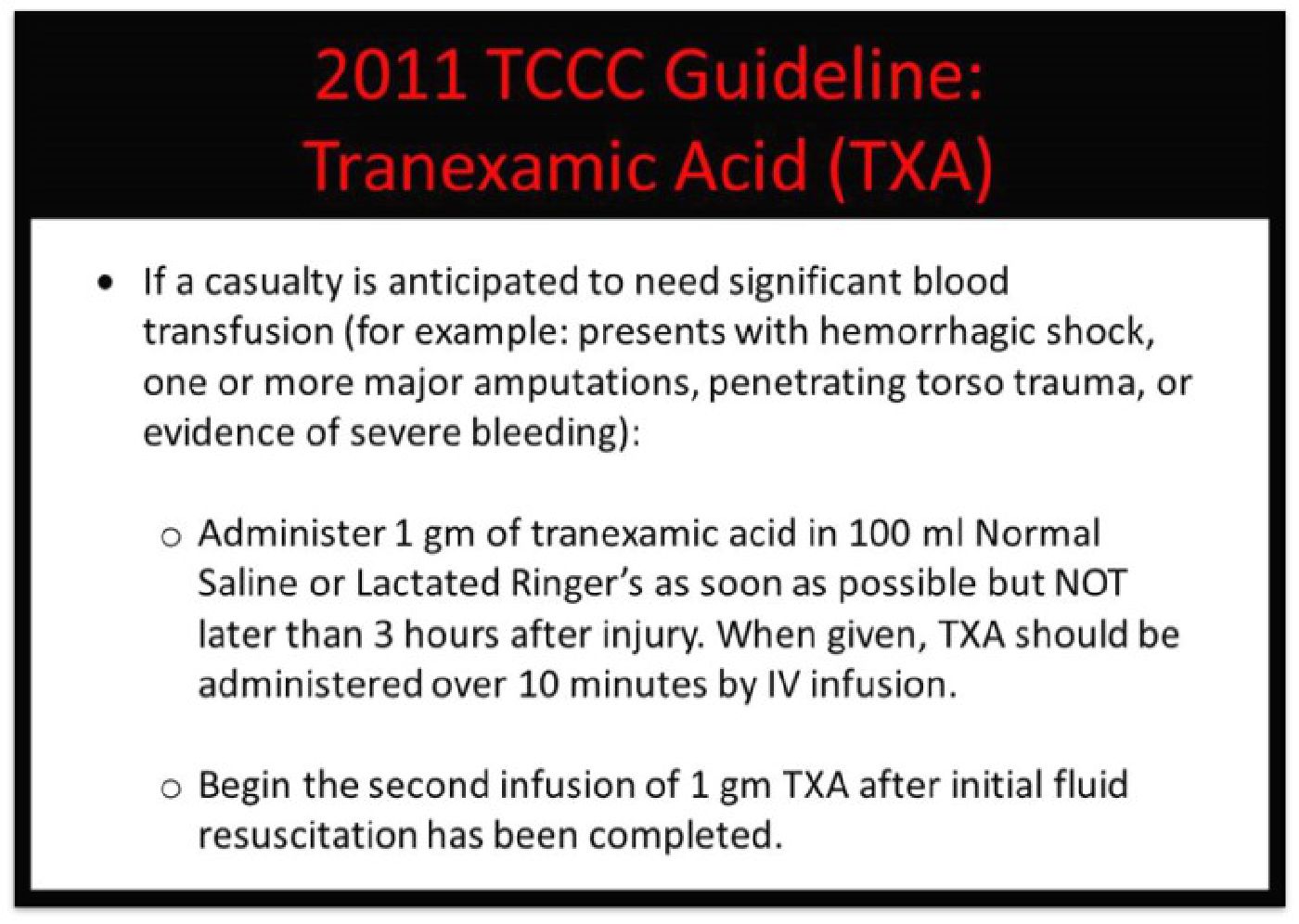
The 2011 TCCC Guidelines reflected TXA's use based on the CRASH-2 trial:
Recent TXA Studies
In a meta-analysis by Gayet-Ageron et al., the authors determined that rapid administration of TXA in hemorrhaging patients improved survival by more than 70%. However, the survival improvement diminished by 10% for every 15 minutes of delay until 3 hours, after which TXA did not confer a survival improvement.
In a study published in conjunction with the Air Medical Prehospital Transport (STAAMP) trial, TXA's usefulness in the pre-hospital setting was reinforced. Guyette et al., in an examination of the STAAMP cohort, determined that TXA's 30-day mortality was lower when tranexamic acid was administered within 1 hour of injury (4.6% vs. 7.6%)."
Research also indicates that TXA is a useful in managing TBI. In a 2019 retrospective review, Chan et al. determined that TXA was associated with lower 30-day mortality in cerebral contusions or traumatic subarachnoid hemorrhage.
The Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage-3 (CRASH-3) trial, completed in 2019, was the largest randomized controlled trial examining TXA's efficacy in isolated TBI. While TXA did not significantly improve 28-day mortality, the authors noted a likely benefit to patients at risk of early mortality. Additionally, Drew et al. noted, the CRASH-3 trial utilized the "Horrow Protocol" rather than a 2-gram bolus of TXA in patients with significant TBI. The working group assessed that the prolonged rate of administration and decreased serum concentration peak produced by the "Horrow Protocol" was insufficient in TBI treatment when compared to a 2-gram bolus.

In 2020, Rowell et al. determined that TBI patients treated with a 2-gram bolus of TXA in the pre-hospital setting had significantly better outcomes than those treated with a 1-gram bolus plus 1-gram maintenance infusion. Patients receiving a 2-gram TXA bolus had a 28-day mortality rate of 18%, while those receiving treatment based on the Horrow Protocol had a 28-day mortality rate of 28%.
CoTCCC Working Group Recommendations
The evidence for TBI patient outcomes was the primary justification for Drew et al. recommending a TXA dose of 2 grams. While data explicitly supporting the use of 2 grams in hemorrhagic shock patients is limited, evidence from the STAAMP trial indicates that a 2-gram dose improved 30-day mortality. While CoTCCC advocates for judiciously monitoring patients for thromboembolism, they do recommend a 2-gram dose regardless of indication, based on recent TBI evidence and the potential benefit for hemorrhagic shock patients.
Feedback from the battlefield indicate that the Horrow Protocol is also logistically cumbersome and has likely decreased the effectiveness of TXA administration. In the Military Application of Tranexamic Acid in Trauma Emergency Resuscitation study (MATTERs), investigators routinely gave TXA via intravenous push without observing significant hypotension. Additionally, military providers have failed to note evidence of significant side effects with the rapid TXA administration. The MATTERs study, military provider experience, and the importance of early administration of TXA in trauma patients support rapid administration (IV/IO push over 1 minute).
While few studies have examined intraosseous administration of TXA specifically, a retrospective analysis of generalized intraosseous access in critically injured trauma patients and feedback from military user groups indicate that intraosseous administration is as effective as intravenous.
In November 2020, CoTCCC adopted the working group’s recommendations and updated approach for TXA's use on the battlefield:

Operational providers have long voiced interest in administering TXA intramuscularly by autoinjector. This route would speed administration and decrease the training and logistical burden required to give TXA. However, a 2018 review found that, while the bioavailability of intramuscularly injected TXA was 100%, it took 60 minutes to reach peak serum levels. This delayed the onset of action of the drug, and the lack of data in trauma patients led CoTCCC to recommend against intramuscular use.
Implications for Wilderness Medicine:
Morbidity and mortality risk due to bleeding is not limited to the military setting, and the risk of traumatic injury is not trivial during outdoor recreational activities. Falls from height, wild animal attacks, trauma during an avalanche, and mountain bike accidents can all deliver injuries that result in significant blood loss and hemorrhagic shock. A retrospective analysis by Flores et al. identified a distribution of injuries that included high rates of injury mechanisms potentially amenable to treatment with TXA. They reported 48% of outdoor injuries resulting from falls, 18% caused by being struck or striking an object, all of which can produce life-threatening blunt trauma. Additionally, the study identified that 6.5% of all diagnosed outdoor recreational injuries were traumatic brain injuries or internal head injuries.
Despite TXA being a relatively safe, inexpensive, and applicable drug to the wilderness medicine setting, there are challenges to its universal use: