In the desperate race to control COVID-19, vaccination against the SARS-CoV-2 virus will be the ultimate containment solution. But for those who have unfortunately fallen seriously ill with coronavirus symptoms, drug and supportive treatment is necessary. Since the novel coronavirus has been with us for a relatively short while, there hasn’t been enough time to develop new drugs. According to the US Food and Drug Administration (FDA)’s Coronavirus Treatment Acceleration Program (CTAP), based on data current as of 8/31/20, much work is being done to develop new drugs. However, there are still no drugs that have been officially approved for COVID-19 prevention or treatment. Research is being performed at breakneck speed all over the globe.
NOTE: After this article posted, the antiviral remdesivir was approved by the FDA on 10/22/20 as a treatment for patients 12 years of age and older hospitalized with COVID-19.
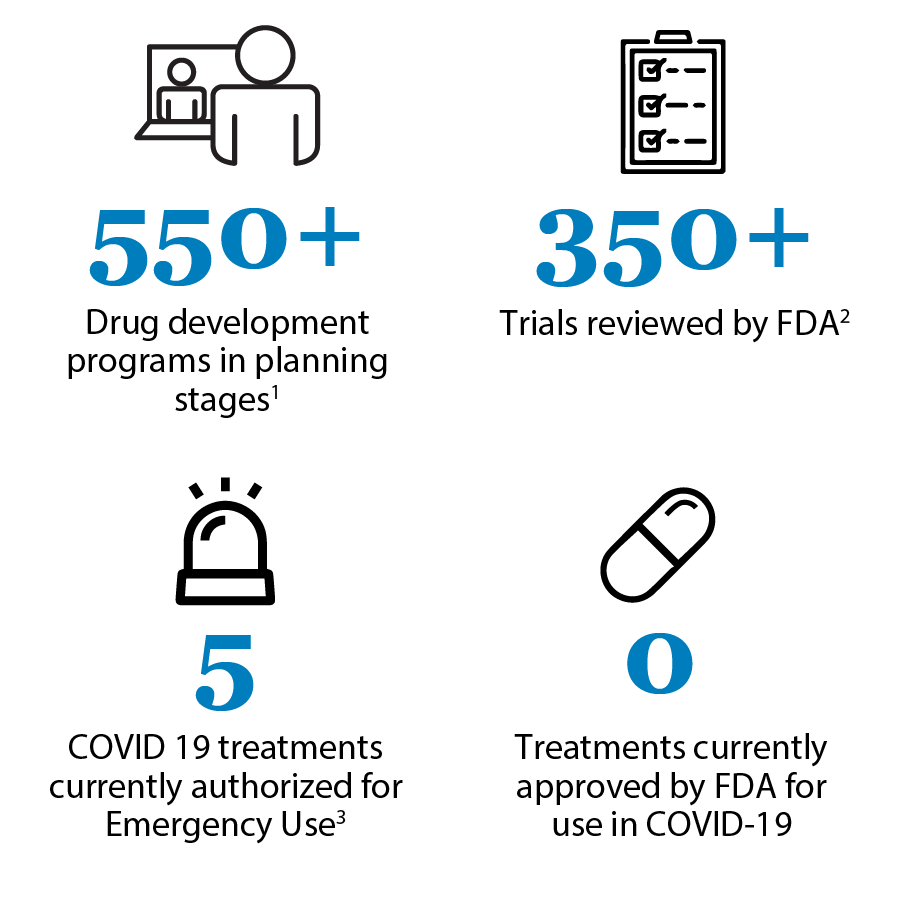
Treatments for COVID-19 focus on two areas: interrupting the virus’ life cycle and controlling symptoms, especially the “cytokine storm” that is responsible for the acute respiratory distress syndrome and other serious and potentially fatal consequences of infection. In the meantime, while vigorous research is being conducted on developing new drugs to kill the virus and control disease progression, scientists and clinicians have turned to currently available drugs using two well known practices: off-label use and repurposing. Emergency use is discussed briefly below as well.
Off-Label Drug Use
Off-label drug use occurs when a medication is prescribed by physicians for indications that have not been approved by the FDA (or a comparable regulatory agency in other countries) or the prescription is for a dose, dosage form, or population for which it has not been approved. “Off-label” means that the use does not appear in the FDA approved drug labeling information. It is rational for clinicians to try a drug for a disease or symptom that is not approved in a certain population, for example pediatrics, pregnancy, or geriatrics, as these populations are often excluded in the original clinical trials. Another reason is for a serious or life-threatening condition such as cancer, where a drug may not be approved specifically for that disease, but it makes pharmacologic sense to try it. COVID-19 falls into this second category. SARS-CoV-2 is a virus, so existing antiviral drugs are being pulled off the shelf and being studied in infected patients to try kill it. In addition, drugs that are not specifically antivirals but interfere with the virus’ life cycle through different mechanisms are being tested. To treat the wide array of life-threatening symptoms caused by cytokine and other inflammatory mediators precipitated by coronavirus infection, older drugs are rationally and sometimes irrationally being tried, gaining widespread media attention in the process.
Repurposing
Repurposing is similar to off-label use. Drugs that are already approved and on the market have been vetted for safety and efficacy, and there is already an available supply, so it makes sense to explore their usefulness during a pandemic caused by a new viral agent. The COVID-19 Early Treatment Fund (CETF), comprised of well known scientists and clinicians from Johns Hopkins, Harvard, Northwestern, University of California, and University of Virginia, was organized to conduct outpatient clinical trials of existing drugs to identify earlier treatments for the disease.
Doctors and researchers at Penn Medicine are also evaluating repurposing drugs for COVID-19 in a program called CORONA (COvid Registry of Off-label & New Agents). In a review of over 9000 patients who received some type of treatment for COVID-19 for the period December 1, 2019 through March 27, 2020, major categories of drugs (Figure a below) and specific drugs (Figure b below) that have been used for COVID-19 were identified. More information on this program can be found at penmedicine.org.