
Pfizer manufacturing plant hit by a tornado in North Carolina July 2023 (click graphic for link)
National security threat!!
Every day, there are threats to national security – global terrorism, espionage, transnational crime, and drug shortages. Wait – drug shortages? This is not something that you think about every day, unless you work in a healthcare setting and experience the frustration of not being able to provide your patients with lifesaving or comfort care.
A government report in late 2019 identified the critical issue of drug shortages in the US – a key risk being an overreliance on foreign manufacturing facilities. An Office of Inspector General report stated that the US Food and Drug Administration (FDA) reported that 74% of active pharmaceutical ingredient (API) manufacturers and 54% of finished goods (what is sold to healthcare facilities and pharmacies) manufacturers are located outside the US. It is estimated that 90-95% of these manufacturing facilities are in China and India. After the COVID-19 pandemic, things only got worse. A follow-up to the 2019 report by the US Senate Committee on Homeland Security & Governmental Affairs in March 2023 announced that there were 295 active drug shortages at the end of 2022, a record five-year high. As of November 3, 2023, there are 128 drugs in shortage. Further adding to national insecurity, the Department of Defense (DOD) stated that it is “wholly dependent” on the commercial market for pharmaceutical products to ensure the “health, safety, and wellbeing of DOD personnel”. Illegal internet-based pharmacies take advantage of shortages by selling counterfeit or illegal versions of drugs, which are purchased by desperate patients who can’t get their essential medications through normal channels.

And of course these shortages are not confined to the US: a report in 2021 of the Organization for Economic Co-Operation and Development (OECD) countries found that 60% of global shortages were due to manufacturing and quality issues, including shortages of materials and suspension of production because of quality issues and technical problems.
What causes drug shortages?
The Federal Food, Drug, and Cosmetic Act defines a drug shortage as a period of time when the demand or projected demand for the drug within the United States exceeds the supply of the drug. In addition to overreliance on foreign sources of medication ingredients, other factors for shortages include supply chain oversight issues and economic drivers. Neither the federal government (eg, FDA) nor pharmaceutical manufacturers have oversight of the entire supply chain – from the sourcing of raw materials and APIs to finished dosage forms obtained by purchasers or providers, scarily leaving the quality of drugs at risk and also rendering the FDA unable to proactively identify when shortages will occur. While the FDA can recall biological products (eg, vaccines), medical devices, and controlled drugs, they are not authorized to require manufacturers to recall most drug products - they can only ask for a voluntary recall. Most of the drugs in shortage are generics, especially parenteral formulations. These are expensive and complex to produce but are cheap to purchase and have narrow profit margins: there is less incentive for domestic pharmaceutical companies to manufacture them. Other factors affect drug production such as national disasters (see photo at top of article of a Pfizer plant destroyed by a tornado in North Carolina) or global conflicts such as the current events in the Middle East or Ukraine – if any drugs or components to make them come from these affected areas, will supply be interrupted?
What drugs are in shortage?
Below are the common classes of drugs that have been in shortage over the past 5 years.
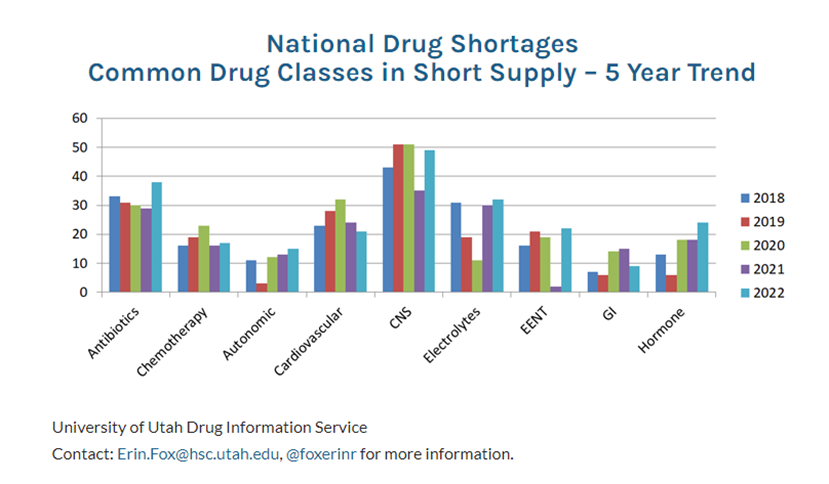
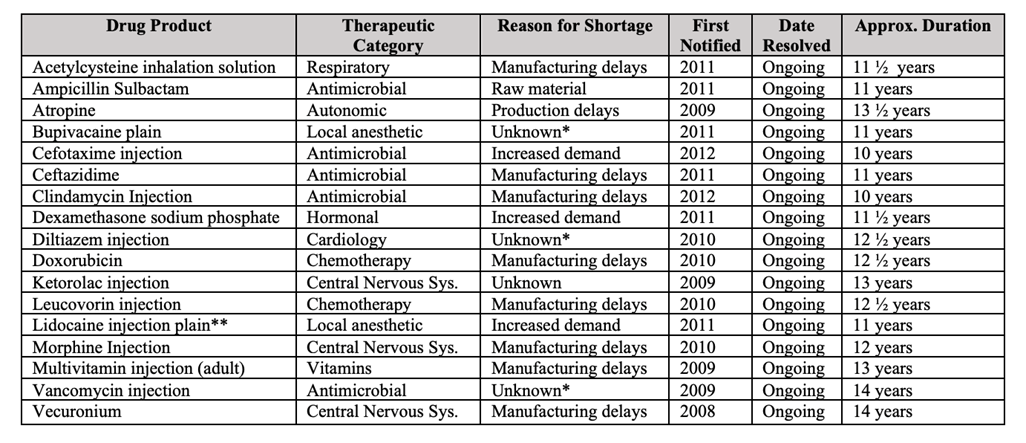
The average drug shortage lasts about 1.5 years, but these are drugs that have been in shortage for over a decade.
Injectables, like IV saline, propofol, and chemotherapy drugs are twice as likely to experience shortages versus oral dosage forms or topicals, but parents may acutely remember the shortage of pediatric ibuprofen (ex., Motrin, Advil) and acetaminophen (ex.,Tylenol) last year. Shortages of over-the-counter drugs can lead to hoarding (remember toilet paper and hand sanitizer at the beginning of the pandemic?). One-third of the shortages are for antibiotics, crucial for combatting bacterial infections. Almost 50% of the antibiotics that are or have been in short supply are on the FDA’s Essential Medicines List (see previous article in this magazine on the EML).
Healthcare providers have admitted to using drugs or equipment in short supply for off-label uses or doses, going beyond beyond-use-dates, and reusing single dose vials. Drug shortages can lead to rising costs for more expensive alternatives, medication waste, medication errors, rationing, delays in treatment – all on top of staffing shortages, resulting in huge frustrations and impact on patient care.
What are possible solutions to the drug shortages problem?
As suggested in the government’s report, solutions to the drug shortage problem include investing more in domestic manufacturing facilities, conducting more thorough assessments of the entire drug supply chain, requiring more reporting by manufacturers of problems they are experiencing, and allowing the FDA to order mandatory recall of problem drug products.
Sources of drug shortages information
The following are key sources on current and resolved drugs and biologics shortages.
American Society of Health-System Pharmacists Drug Shortages
FDA Drug Shortages (main page)
FDA Drug Shortages (FAQs)
FDA Biologics Shortages (vaccines, blood, immune globulins, etc.)
FDA 2019 Drug Shortages Report
FDA Extended Use Dates (to assist with shortages)
Institute for Safe Medication Practices
End Drug Shortages Alliance
So how do drug shortages impact wilderness medicine?
While most of these shortages impact practitioners in hospitals (especially the ED) or pharmacies, there are products with wilderness or travel medicine applications that have been affected. Some of these drugs appear on the current FDA page and ASHP page:
-albuterol sulfate solution
-amoxicillin suspension
-atropine sulfate
-bupivacaine, bupivacaine/epinephrine
-dexamethasone injection
-diazepam rectal gel
-epinephrine injection 0.1mg/ml
-epinephrine autoinjectors
-hydrocortisone injection
-ketamine injection
-lidocaine injection
-methylprednisolone injection
-morphine sulfate injection
Black widow spider antivenom: when I worked at Merck, this antivenom was in shortage and on allocation for years. If a call came in the wee hours, the product had to be driven to the patient’s facility under the cover of night. Not pleasant for those who got pierced by the widow’s fangs (although supportive care was often the best treatment). The shortage has been resolved at long last.
Snake antivenoms: there has been a longstanding dire lack of supply worldwide of these difficult to produce biologic products, especially for more “exotic” species in developing nations.
Vaccines: while there are currently no routine vaccines that are in short supply, there have been a number of travel vaccines that have been in and out of supply or allocation, creating much consternation. Yellow fever vaccine (YF-Vax) was not available for 4 years in the US (and longer in some parts of the world) and a process had to be put in place to obtain an ex-US version of the vaccine during the shortage. An alternative fractional dosing schedule was also recommended to extend supplies globally, although this was not ideal. BCG vaccine, used globally for prevention of tuberculosis and treatment of bladder cancer, has been in short supply, as well as the new mpox vaccine. Prior shortages of travel-related vaccines such as rabies, cholera, typhoid, and hepatitis B vaccines have been resolved, but if makes you wonder if it will happen again.
The ASHP site is good about offering any available alternatives to drugs in shortage, so make sure to check it before you assume you won’t be able to obtain that med to pack in your expedition kit.