It makes sense for medications to be stored at room temperature or “conditions of civilization” (1) – but of course not all medications may be stable at 20º-25ºC (68º-77ºF). Or they may be susceptible to moisture, light, or other conditions that aren’t the perfect environment in which the pharmaceutical company that manufactured them originally tested them for stability.
First, let’s think of how you store your medications at home. Do you keep them in the original bottle from the pharmacy or do you throw all your pills together in one bottle so you don’t have to open different bottles every day? Do you correctly store medications that have to be refrigerated in the fridge? Do you even know at what temperature your refrigerator is set? If you have medications mailed to you, do they sit in your mailbox on a 100-degree day until you get home from work? Or are they buried in the snow on your porch step on a 5-degree day? Are they stored in your medicine cabinet in the bathroom – which gets hot and steamy every time a shower is taken? Are they sitting on the kitchen windowsill so you won’t forget to take them, exposed to direct sunlight?
In wilderness and remote environments, which may be perfect for an exhilarating experience but not for drug storage, conditions may be much more extreme. Are you trekking in the Mongolian desert toting medications, whether for yourself or for an expedition? Climbing a 20,000-foot peak? Did you pack the medications in your checked-in luggage so they’re sitting in the freezing non-pressurized cargo area in the plane? Are your pills rolling around in a bottle on a rocking dive ship?

HOW DO YOU KNOW AT WHAT TEMPERATURE A MEDICATION NEEDS TO BE STORED?
First, here’s the conversion between °C and °F:
°F = °C x 1.8 + 32
°C = (°F-32) x 5/9 (0.56)
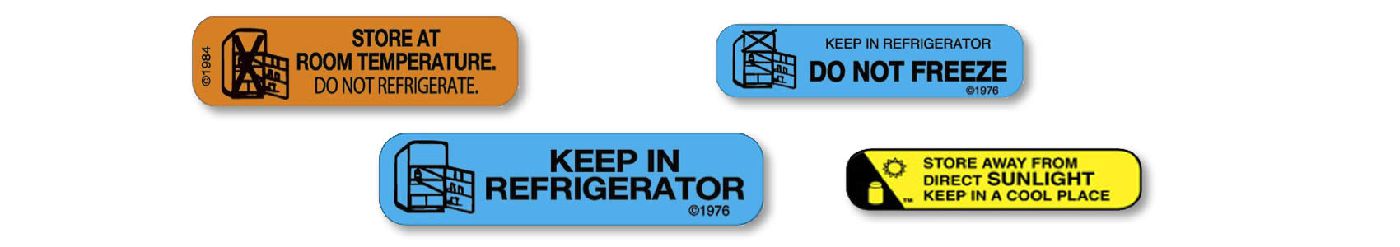
The recommended storage range is pretty simple for over-the-counter drugs: it’s printed on the container or blister pack:

However, for prescription drugs, unless they are dispensed in the original manufacturer’s container, the amber or white plastic bottle the pharmacy dispenses does not usually contain the proper storage range unless the drug needs to be stored at a particular temperature, for example, refrigerated, protected from light, etc.
The best way to find the correct storage temperature for prescription drugs is in the manufacturer’s prescribing information (found on the drug company’s website, the PDR, or a copy that is provided by the pharmacy). A typical “Storage and Handling” section for a room temperature product reads:
Store at 20-25°C (68-77°F), excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature.]
A typical section for a refrigerated product reads:
Store refrigerated at 2 to 8°C (36 to 46°F).
If appropriate, it may also contain:
Do not freeze. Protect from light.
Fortunately, not many products need to be frozen, which presents another level of complexity in environments outside the home or healthcare facility.
CONDUCT OF STABILITY STUDIES BY THE MANUFACTURER AND SIMILARITIES TO THE WILDERNESS ENVIRONMENT
Drug manufacturers adhere to stability testing guidelines put forth by the United States Pharmacopeial Convention (USP), International Conference on Harmonization (ICH), or the World Health Organization (WHO). Drugs are tested for stability under long-term and accelerated storage conditions, and compatibility with the container they will be shipped in. If temperature controls are needed during distribution of the drug (i.e., “cold chain”) and these are not met, for example if there are transport delays and extreme temperatures, then a temperature excursion occurs, possibly putting the drug at risk of losing its efficacy or even becoming toxic. (2)
One can think of transport of a drug in a wilderness medicine or travel setting as somewhat analogous to this production, testing, and distribution of the drug by the manufacturer (“supply chain”).
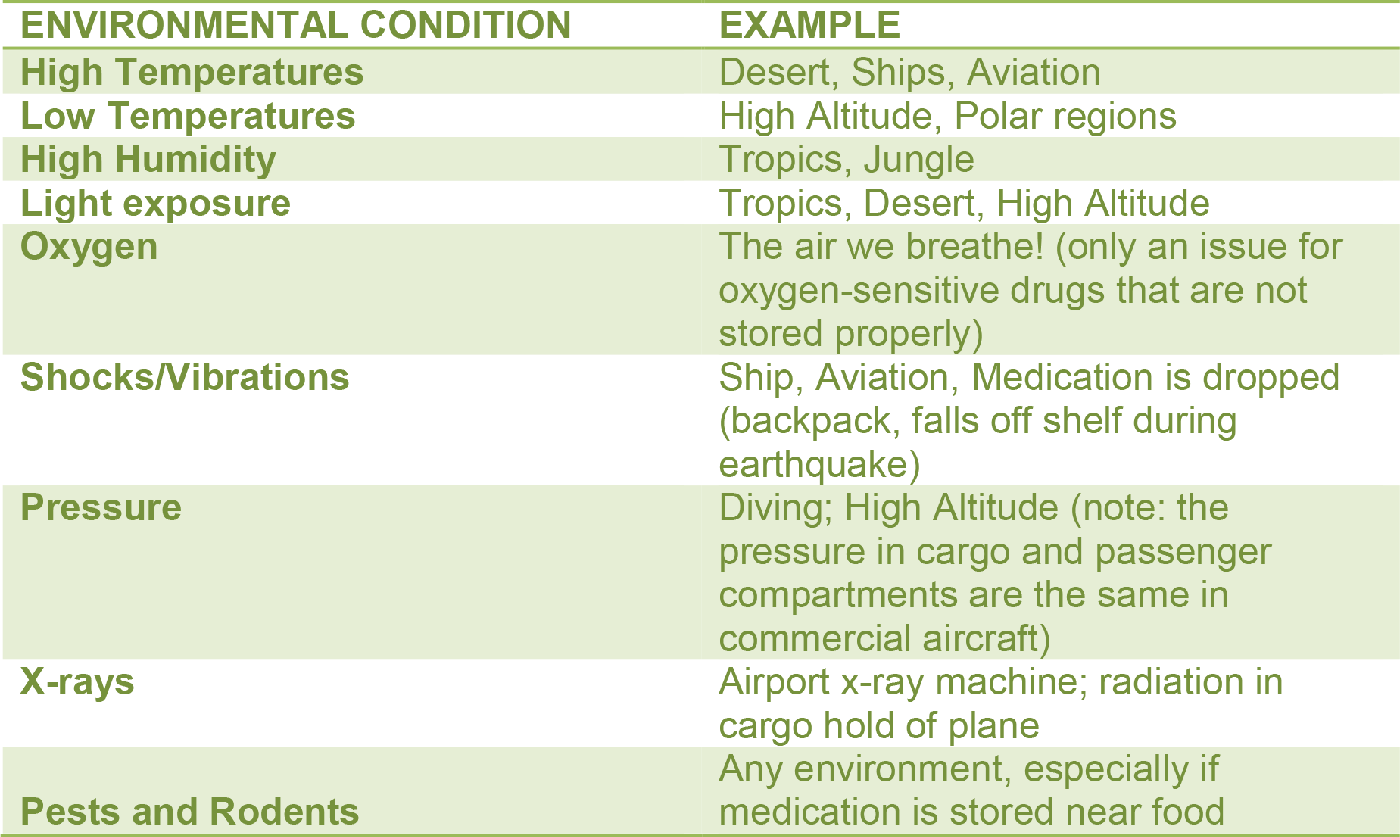
Below are environmental conditions to which a drug can be exposed during transport by the wilderness traveler:
WHAT HAPPENS TO DRUGS WHEN THEY ARE NOT STORED IN PROPER CONDITIONS?
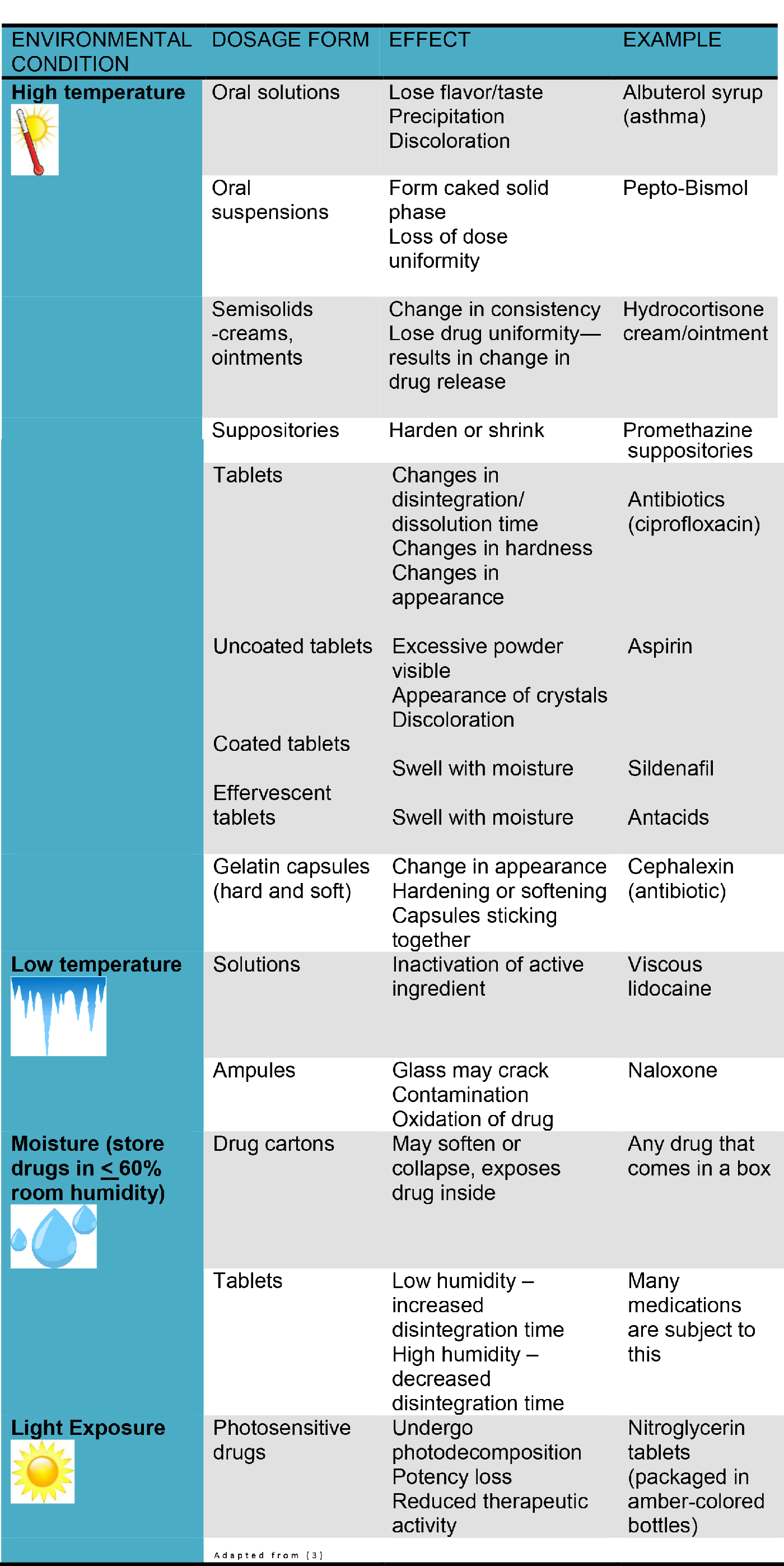
The following table illustrates different types of environmental conditions and what can happen to medications, with an example of a medication that would be used in wilderness medicine.
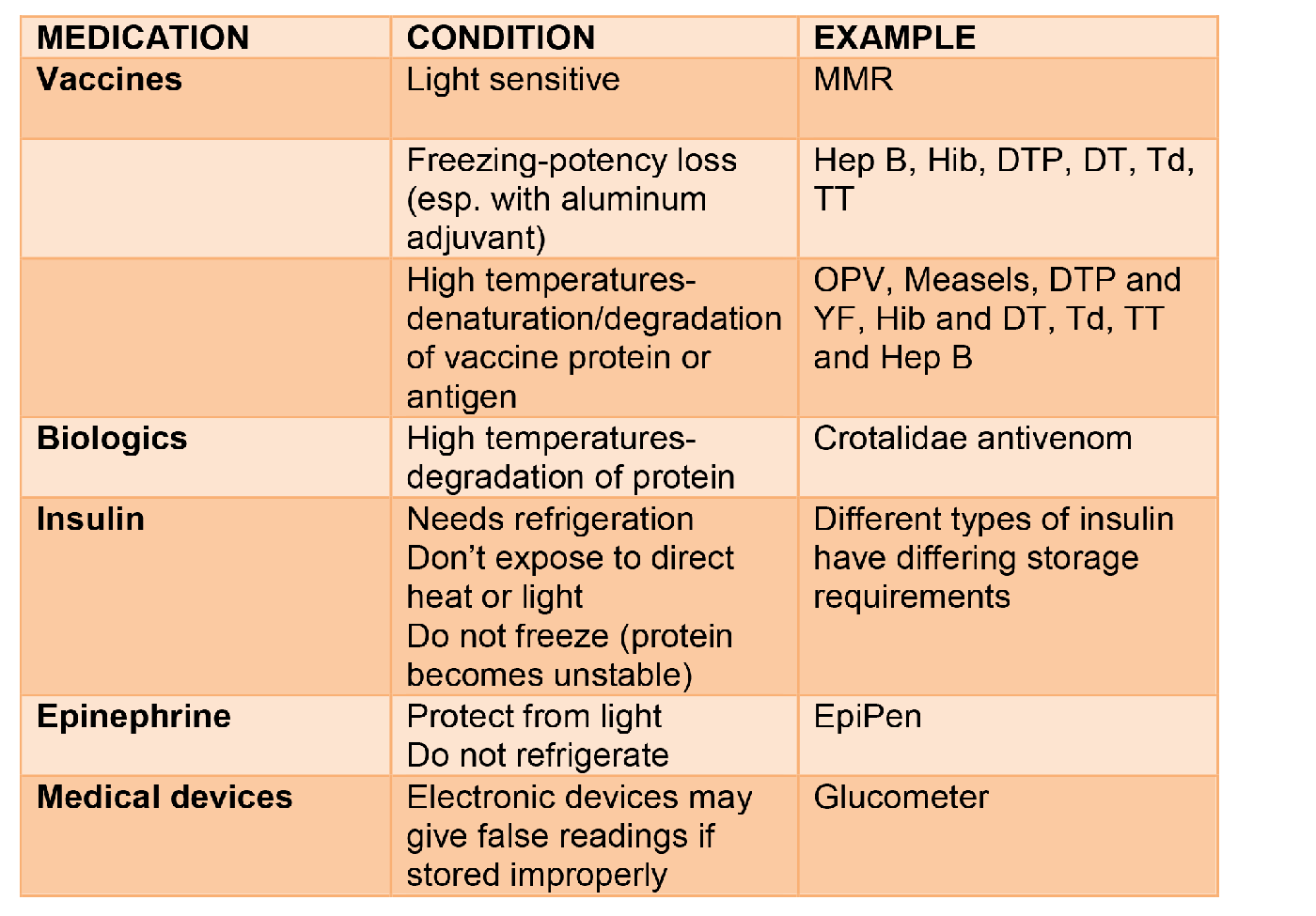
The following are other medications that may be used in wilderness or travel medicine and possible effects of the environment on stability.
MEDICAL LITERATURE ON STABILITY OF MEDICATIONS IN EMS, WILDERNESS, OR EXTREME ENVIRONMENTS
EMS
Brown and Campagna did a very nice review in EMS World about 10 years ago on storage of medications in the EMS environment. They included the table below with guidelines from the USP for storing medications in EMS vehicles and ambulances. (4)