Why Do Drugs Have An Expiration Date?

The question of whether drugs can be used past their expiration date regularly makes the rounds on the news and internet. Information and responses range from “NO” to “Cipro is good for up to 11 years after its posted expiration date!!!!” Conspiracy theories abound as to why expiration dates appear on medications, but the reason is fairly simple: in the United States in 1979, the FDA started to require drug companies to put an expiration date on their products. The expiration date is a guarantee by the manufacturer of the safety, potency, and stability of the product up until that date (the drug must be at least 90 percent of the original potency). Importantly, this applies to drugs in the original container and stored per the manufacturer’s recommendations.1

The expiration date is defined as “the date placed on the container/labels of a drug product designating the time during which a batch of the product is expected to remain within the approved shelf life specifications if stored under defined conditions, and after which it may not be used.” (21 CFR 211.137, 211.166). (Note: “shelf-life,” also referred to as “expiration dating period,” is defined by the FDA as the time period during which a drug product is expected to remain within the approved shelf life specification, provided that it is stored under the conditions defined on the container label).2 When a manufacturer submits a New Drug Application to the FDA, it includes a stability assessment of the new drug product (which includes the active pharmaceutical ingredient [API] and excipients) in the packaging intended for final sale. Stability testing includes long-term (storage at 25°C +/- to 2°C/60% RH [relative humidity] +/- 5% RH for 12 months minimum) and accelerated stability testing (storage conditions 40°C +/- -2°C /75% RH+/- 5% RH for 6 months). The typical initial expiration dates for prescription products are usually 18-24 months, and all products pretty much fall into the range of 12-60 months.3
How Do You Read An Expiration Date on a Medication Container?
Even though the CFR specifies that expiration dates must be put on medication bottles or other types of packages, they don’t specify how they have to be expressed. Does “05/16” mean May 1, 2016 or May 31, 2016? Does “04/03/17” mean April 3, 2017 (dating convention in United States) or March 4, 2017 (dating convention in European Union and other countries)? What does “JN18” mean? (January or June 2018? The first or last day of the month?) There have been efforts by groups like the US Pharmacopeia (USP) and Consumer Healthcare Products Association (for OTC drugs) to improve the clarity of expiration dating for both healthcare professionals and consumers as well as to help avoid medication errors.

From: http://www.consumermedsafety.org/safe-medicine-storage-and-disposal/what-does-a-medicine-s-expiration-date-mean
Expiration dates are not the same as “beyond-use” dates (BUD). Once a drug product is removed from the manufacturer’s container and is repackaged into a different container (e.g., into an amber pharmacy bottle, unit dose packages for hospitals), the USP specifies that a beyond-use date be placed on the label of the new packaging; this is the date after which a drug product should not be used. The BUD should be no later than the expiration date on the original manufacturer’s container or one year from the date the drug is dispensed, whichever is earlier.
Are Drugs Still Potent After They Pass Their Expiration Date?
Conspiracy theory: shorter expiration dates are a way for drug companies to sell more drugs even though the drugs are stable for many years past their expiration date. Pharmaceutical companies may state that drugs can maintain some potency after the expiration date, but stop short of recommending their use after the date has passed. How soon does the clock start ticking on loss of drug potency after the expiration date?
There’s a good reason why it’s important to know if certain drugs retain potency after the labeled manufacturer’s expiration date. The Strategic National Stockpile, overseen by the CDC, is a large supply of drugs and medical supplies used to protect American citizens in the event of a public health emergency like a terrorist attack, influenza outbreak, earthquake, etc.)4 If these medical countermeasures (MCMs) reach their expiration date before being used, they must be discarded, which can be very expensive. To help prevent the loss of potentially still potent medication, the FDA is involved in several expiration dating programs. The Shelf-Life Extension Program (SLEP) was established in 1986 by the US Department of Defense to conduct extended stability studies on a number of drugs in federal stockpiles like the SNS.5 In 2006, a summary report was issued on 122 different drugs (>3000 lots). It was found that 88 percent of the lots were extended by at least a year past their original expiration date (the average extension was ~5.5 years). Top performers included ciprofloxacin tablets (yes, almost 12 years!), naloxone HCl, and potassium iodide (23 years!), with many year extensions for multiple drugs past their labeled expiry.6
Another program is the Emergency Use Authorization (EUA) for CBRN (chemical, biological, radiological, and nuclear defense) emergencies. Under this program, the FDA can approve the use of a product (that would typically be used in a CBRN emergency) beyond its labeled expiration date, provided that the product has been proven to be safe. An example is the “Doxycycline EUA Fact Sheet for Recipients,” given to persons exposed to anthrax. The sheet contains the following statement: “If you have received doxycycline with an expired date on the package, FDA has authorized its use. Testing of the medicine found it is safe to use past the expiration date.”7

The Effect of the Formulation and Environment on a Drug’s Expiration Date
The extended stability studies discussed above have been conducted on drugs in their original containers and stored according to the manufacturer’s recommendations. Storage at extremes of temperature (hot or cold), exposure to oxygen, moisture (high humidity), or exposure to light may affect the potency of drugs, and therefore the true potency may not be reflected in the expiration date. In general, most drugs are most stable when stored at colder temperatures than warmer temperatures than recommended, although adherence to the manufacturer’s recommended storage range in the prescribing information (or on the package carton for OTC products) is the best way to ensure potency. Solid dosage forms (tablets, capsules) are much more stable than solutions or suspensions and injectable formulations.
Can Drugs Become Harmful After They Expire?
According to the Medical Letter,8 there are no reports on current medications regarding the toxicity of using a drug past its expiration date, whether taken orally, by injection, or topically. For years, it was well known that taking outdated tetracycline caused Fanconi Syndrome (renal proximal tubular damage) due to the degradation products epitetracycline and anhydrotetracycline. The ML states that this formulation is no longer available. However, it is important to note that toxicity of expired medications is an area that is not well researched.
Which Drugs Should Definitely Not Be Used Past Their Expiration Date?
Any drugs that become discolored, powdery, or are emitting an odor should not be used. Drugs that are injectable solutions should not be used if they appear cloudy or have precipitates. The table below lists some drugs that should not be used past their expiration date.
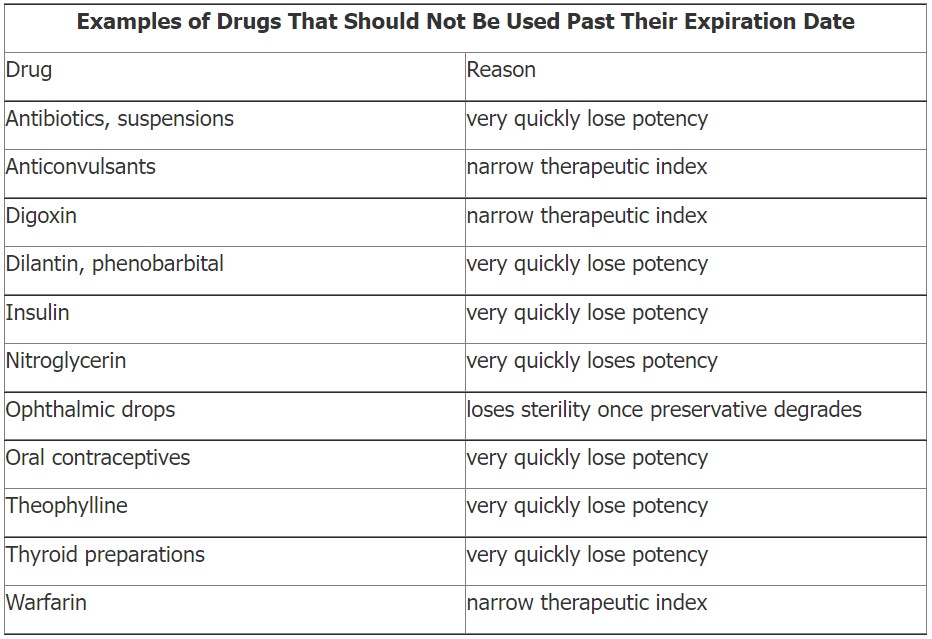
Adapted from: http://www.emedexpert.com/tips/expired-meds.shtml
Epinephrine is of special importance to the wilderness medicine community.
In SLEP studies, epinephrine in a cartridge-needle (brand not specified) failed ~50 percent of lot extension expiration evaluations. An oft-quoted prospective, randomized, cross-over study in rabbits of administration of expired EpiPen and EpiPen Jr. found a significantly reduced epinephrine bioavailability after injection of the expired pens (1-90 months) versus those that were in-date. The decrease was proportional to the months past the expiration date. However, the authors concluded that if an expired EpiPen is all that is available during an anaphylactic reaction, it is reasonable to use it if there are no discolorations or precipitates in the solution.9
Can Expired Drugs Be Donated for Medical/Humanitarian Relief Efforts?

What if you are going to an earthquake-ravaged country on a disaster medical relief mission and want to bring some newly or nearly expired medication with you? The U.S. Transportation Security Administration (TSA) offers clear guidelines on how to travel with drugs: “Prescription medications should be in their original containers with the doctor’s prescription printed on the container. It is advised that you travel with no more than a 90 day supply. If your medications or devices are not in their original containers, you must have a copy of your prescription with you or a letter from your doctor.”10
It’s not clear what is meant by “original containers,” but likely refers to the prescription bottle. As discussed above, once drugs are out of the original manufacturer’s container, the labeled expiration date cannot be guaranteed, so special care needs to be taken to store the product as close to the recommended conditions as possible, especially if a supply of medication is to be left in the affected country which may have extremes of temperature or improper storage facilities (see “Drug Stability in the Wilderness” Appendix in Wilderness Medicine, 6th Edition).
For relief organizations, the World Health Organization (WHO) and the FDA have clear guidelines on donating expired or nearly expired drugs for humanitarian relief efforts. According to the FDA’s “Questions and Answers for the Public Donating Drugs to International Humanitarian Relief Efforts:”11
FDA and the World Health Organization (WHO) strongly discourage donation of expired drugs, even to affected nations during an emergency or crisis. Additionally, nations may consider the dispensing of expired drugs to be illegal, including during a crisis. Guidelines from the WHO state that drugs with less than one year before their expiration date will automatically be destroyed.12 Depending on the nature of the emergency, and on a case-by-case basis, FDA is prepared to exercise enforcement discretion on the exportation of a designated product as long as it is to fulfill a request from the importing country and found acceptable by that country; and the expiration issues have been evaluated by an appropriate component of FDA, or when reasonable assurance is provided to the receiving country and FDA that the product meets quality specifications. The FDA would not object to the donation of drugs that are past or within one year of the expiration date shown on the label when provided with sufficient information to show the expired lot(s) are safe and effective. Furthermore, the FDA expects that no U.S. marketplace shortage would occur due to the exportation of the donated drugs, and that within expiry supplies would be preferentially used before the expired or near expiration supply, if practical. FDA has and will allow the use of a drug under similar circumstances when needed to alleviate a U.S. shortage.
So, are expired drugs immortal or should they be considered DOA once they have reached their expiration date? Depends.
References:
- CFR Code of Federal Regulations Title 21. US Food and Drug Administration Website http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=211.137]
- http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073369.pdf
- http://www.ama-assn.org/meetings/public/annual01/csa_reports.pdf
- http://www.cdc.gov/phpr/stockpile/stockpile.htm
- https://slep.dmsbfda.army.mil/
- Lyon RC, Taylor JS, Porter DA, Prasanna HR, Hussain AS. Stablity Profiles of Drug Products Extended beyond Labeled Expiration Dates. J Pharm Sci 2006;95:1549-1560.
- http://www.fda.gov/downloads/EmergencyPreparedness/Counterterrorism/UCM265824.pdf
- Drugs Past Their Expiration Date. Med Lett Drugs Ther 2009;51:101-102).
- Estelle F, Simons R, Gu X, Simons KJ. Outdated EpiPen and EpiPen Jr autoinjectors: Past their prime? J Allergy Clin Immunol 2000;1025-1030.
- https://help.cbp.gov/app/answers/detail/a_id/67/~/traveling-with-or-mailing-medications-and-medical-devices,-such-as-needles-or
- http://www.fda.gov/downloads/NewsEvents/PublicHealthFocus/UCM249617.pdf
- http://apps.who.int/medicinedocs/pdf/whozip52e/whozip52e.pdf
Posted on November 29, 2015