This is Part III of a four-part series detailing some highlights of early diving physiology and medicine. See Part II in this series, Technological Advances in Underwater Air Delivery, for a brief overview of air delivery to divers from the late 18th to the mid-19th century. Part I, Incipient Underwater Efforts, provided a brief overview of some of the earliest human explorations of the underwater world.
It was not until the 1870s that decompression sickness started to be studied scientifically, most notably by the French physiologist Paul Bert (1833-1886, Fig. 1 and Fig. 2) who subsequently published many of his results in the monumental book La Pression Barométrique: Recherches de Physiologie Expérimentale, later translated to English as Barometric Pressure: Researches in Experimental Physiology. This magnum opus deals with not only physiological effects pertaining to operation in environments of high atmospheric pressure, but in low atmospheric pressure (i.e., hypobaric high altitude) environments as well. Of particular relevance to the hyperbaric environment, Bert was able to experimentally demonstrate that rapid decompression was in fact associated with the formation of gas bubbles consisting mainly of nitrogen. He was also the first to show the rapid toxic potential of pure oxygen when breathed under pressure.

Figure 1 - Paul Bert, from Barometric Pressure: Researches in Experimental Physiology
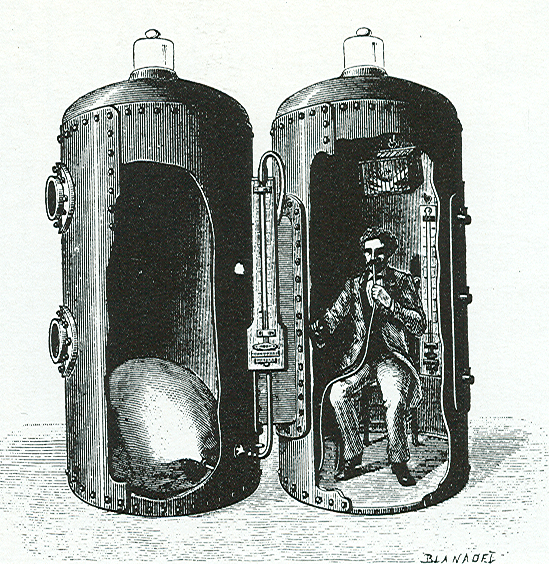
Figure 2 - Paul Bert pictured inside his largest hyperbaric/hypobaric chambers (built to accommodate humans), from Barometric Pressure: Researches in Experimental Physiology
It is of no small significance to realize that Bert’s landmark discovery of the hazards of rapid decompression essentially occurred by accident – during experiments on transition to low barometric pressure that were attempting to simulate physiological adaptation to high altitude. Bert realized that low altitude dwellers often require many days to fully acclimatize to high altitudes. In the process of running tests of what physiological changes occur in small animals during slow chamber decompression to a simulated high altitude, an accidental decompression and subsequent death of an otherwise healthy sparrow took place. Gross findings on autopsy did not reveal an obvious cause of death, but Bert suspected he had stumbled upon an important finding.
When nearly two hundred years earlier Robert Boyle had noted bubbles within the eye of a snake he had decompressed from 1 atmosphere of pressure, it was not possible to further investigate the mechanisms of bubble formation since the chemical revolution that would have made this possible did not occur until the latter half of the eighteenth century. With the chemical revolution came a reformulation of chemistry based on the Law of Conservation of Matter and the oxygen theory of combustion, and centered on the work of French chemist Antoine Lavoisier. Several factors led to this revolution, such as proof that air was not an element but was composed of seven different gasses. Paul Bert benefitted not only from the scientific happenings of the eighteenth century, but from the technical advances and improvement in laboratory techniques that characterized scientific progress in the early to mid-nineteenth century as well. These improvements meant that Bert was able to measure gases in the blood of his experimental animals with absorbents by a process called “absorbent volumetry” developed in the 1850s by German chemist W.E. van Bunsen (of burner fame).
[Bert] modified Bunsen’s gas analysis method by constructing a mercury-filled pump that allowed blood extraction from a pressurized animal. The blood samples would be pumped and the evolved gases analyzed with the absorbent method. After the addition of potash, carbonic acid (carbon dioxide) was absorbed and then pyrogallic acid, which absorbed oxygen. The remaining gases, by inference, would be nitrogen.
It was not until the 1890s and the introduction of the spectrophotometer that it became possible to measure nitrogen directly. Nonetheless, Bert realized that even with 1870s-era gas analysis methods
…the gas which would threaten life on being liberated would be exclusively the one the proportion of which was considerably increased in the blood, that is, nitrogen…[Bert noted that unlike oxygen and carbon dioxide] nitrogen does not escape through the lungs because it is in an atmosphere which is four-fifths nitrogen and nothing urges it out [and he thus theorized] the tissues of the organism…are laden [during compression] with a growing proportion of nitrogen …and when decompression occurs, the gases must necessarily return to a free state, distending and even lacerating the tissues from which they escape…We therefore understand the risks run by workmen [in compressed air environments] whose paralysis or death…depends upon the size of a bubble of gas.
Bert’s discovery that nitrogen was the gas responsible for decompression sickness represents a milestone in the history of physiology. However, Bert realized there were still very important questions to be answered regarding the phenomenon we have come to commonly refer to as the “bends”. Among the outstanding questions to be answered was why different animals – even those of the same species – that were decompressed from the same pressure in the same length of time would manifest such different symptoms and rates of mortality. Such heterogeneity of decompression illness-related symptoms was fuel for Bert’s critics, especially as it concerned his nitrogen bubble hypothesis. In particular, many found it incredible that the same evolved nitrogen bubbles that were killing animals decompressed from 10 or more atmospheres of pressure were also causing morbidity and occasionally mortality in caisson workers who were decompressing from as little as 2 atmospheres. Thirty years would pass before this matter was satisfactorily answered.
Nitrogen was, however, not the only gas of interest to Bert. Soon after the discovery of oxygen, one of its discoverers, Joseph Priestly, had theorized about the effects of hyperoxia:
We may also infer from these experiments, that though pure dephlogisticated air might be very useful as a medicine, it might not be so proper for use in the healthy state of the body: for, as a candle burns out much faster in dephlogisticated than in common air, so we might, as might be said, live out too fast, and the animal powers be too soon exhausted in this pure kind of air. A moralist, at least, may say that the air which nature has provided for us is as good as we deserve.
Bert’s 1878 book, La Pression Barométrique, provided the first actual documentation of oxygen toxicity -- on a variety of life forms. Bert noted that seizures were not an uncommon central nervous system (CNS) manifestation of oxygen toxicity, and this phenomenon has subsequently come to be commonly known as the “Paul Bert effect”. Such seizures are associated with short-duration, high partial pressure exposures to oxygen as may occur with diving. Pulmonary oxygen toxicity associated with prolonged exposures at lower partial pressures (e.g., at or near normobaric pressures), however, was missed by Bert. This was later discovered by Lorrain Smith just before the turn of the twentieth century, and is, not surprisingly, often referred to now as the “Lorrain Smith effect”. Unlike the CNS pathology associated with the Paul Bert effect, the pulmonary pathology seen with the Lorrain Smith effect is often apparent when patients breathe (uninterrupted) pure oxygen under normobaric conditions for more than 12 hours or so. This has been noted in autopsy studies of critically ill patients exposed to prolonged artificial (mechanical) ventilation at a high fraction of inspired oxygen. As a result of this “type” of oxygen toxicity, the lung alveoli will start to leak a protein-rich fluid, and the subsequent pathophysiological chain of events related to edema collection will ultimately lead to atelectasis.
The final portion (Part IV) of this series on the early history of diving physiology and medicine will focus on John Scott Haldane’s contributions to the understanding of high pressure physiology and the prevention and treatment of decompression pathology in the early 20th century.