Introduction
Travel to elevations above 2500 m is associated with risk of developing one or more forms of acute altitude illness: acute mountain sickness (AMS), high altitude cerebral edema (HACE), and high altitude pulmonary edema (HAPE). Although unacclimatized individuals are at risk of high altitude illness when ascending to altitudes above 2500 m, it is possible to see acute altitude illness present at lower elevations due to a high degree of variability in responses to high altitude between individuals.
To provide guidance to clinicians and disseminate knowledge about best practices, the Wilderness Medical Society (WMS) convened an expert panel to develop evidence-based guidelines for prevention, diagnosis, and treatment of acute altitude illness. Preventive and therapeutic modalities are presented, and recommendations made for each form of acute altitude illness.
Acute Mountain Sickness and High Altitude Cerebral Edema
From a clinical standpoint, HACE represents an extremely severe form of AMS; therefore, preventive and treatment measures for the two disorders can be addressed simultaneously.
AMS/HACE Prevention
Suggested Approach: The approach to prevention of AMS and HACE should be a function of the risk profile of the individual traveling to high altitude (Figure 1). The first priority should be ensuring gradual ascent to the target elevation. Travelers can lower their risk by sleeping one night at an intermediate altitude. For example, sea level residents traveling to Colorado resort areas over 2800 m can spend one night in Denver (1600 m). If travelers are unable to ascend gradually due to various logistical factors, pharmacologic prophylaxis can be considered. Prophylactic medications are generally not necessary in low risk situations but should be considered in addition to gradual ascent for use in moderate to high risk situations (Figure 1). With travel above 3000 m, individuals should not increase the sleeping elevation by more than 500 m per day and should include a rest day (i.e. no ascent to higher sleeping elevation) every 3-4 days.
Acetazolamide: Acetazolamide has an established role in prevention of AMS. There is a risk, albeit extremely low, of inciting an allergic reaction in persons with sulfonamide allergy when taking acetazolamide, and the drug is contraindicated in persons with any prior anaphylactic reaction to a sulfonamide medication or a history of Stevens-Johnson syndrome. Acetazolamide should be started the day before ascent but will still have beneficial effects if started on the day of ascent (Table 1).
Dexamethasone: Dexamethasone does not facilitate acclimatization like acetazolamide but has an established benefit in AMS prevention. Higher doses than those recommended in Table 1 (4 mg every 6 hours) may be considered only in very high risk situations such as military or search and rescue personnel being airlifted to altitudes > 3500 m with immediate performance of physical activity. If used for longer than 5-7 days, dexamethasone should be tapered over a one week period rather than stopped abruptly. It is not recommended for AMS prevention in children. Dexamethasone should be started the day before ascent but will still have beneficial effects if started on the day of ascent.
Ibuprofen: Ibuprofen cannot be recommended over acetazolamide or dexamethasone for AMS prevention during rapid ascent (Table 1), however, it could be used in persons who do not wish to take acetazolamide or dexamethasone or have allergies or intolerance to these medications.
Staged Ascent and Preacclimatization: The panel does not endorse a particular protocol for preacclimatization (i.e., repeated exposures to hypoxia preceding high altitude travel) and staged ascent (i.e., staying at a moderate elevation for several days before ascending to the target elevation) as a means of AMS prevention. With regard to normobaric hypoxia exposure in a hypoxic tent, only sufficiently long exposures (> 8 hours per day) that can be undertaken regularly over an appropriate number of weeks are likely to offer any significant preacclimatization benefit.
Other Options: The following interventions have not been shown to be of benefit: chewed coca leaves, coca tea and other coca-derived products; “forced” or “over” hydration; short-term oxygen use in the form of either visits to oxygen bars or over-the-counter oxygen delivery systems by which individuals inhale oxygen-enriched gas from a small pre-filled canister; other over-the-counter products, such as powdered drink mixes, patches, and oral supplements.
AMS/HACE Diagnosis
The diagnosis of AMS is made in a very specific clinical context, that of an unacclimatized lowlander who becomes ill within several hours to 3 days following ascent to high elevations, generally >2500 m. Diagnosis is based solely on reported symptoms, as there are no characteristic findings on physical exam or diagnostic laboratory studies. Symptoms of AMS include: headache, anorexia, nausea, fatigue, and light-headedness or dizziness. In line with the updated Lake Louise Acute Mountain Sickness Score (see Table 2), these guidelines emphasize the role of headache and de-emphasize the role of sleep disruption in the diagnosis of AMS. The best approach to diagnosis is to consider the traveler’s well-being and functional status. Individuals who feel ill and/or must reduce their daily activities several hours to 3 days following ascent to elevations >2500 m likely have AMS.
The diagnosis of HACE is heralded by signs of encephalopathy including ataxia–which is often the earliest clinical finding–and altered mentation. Other signs include apathy, irritability, lassitude, and inability to provide self-care, all of which can be subtle. Untreated HACE can progress to coma. Focal neurologic deficits are unusual and should prompt consideration of other diagnoses. Heel-to- toe walking can be used to gauge ataxia, and papilledema can confirm cerebral edema.
AMS/HACE Treatment
Suggested Approach: Care should be taken to exclude disorders whose symptoms and signs resemble those seen with AMS and HACE, such as cerebrovascular accident, carbon monoxide poisoning, hypoglycemia, hyponatremia, infection, or traumatic brain injury.
Persons with AMS of any severity or HACE should cease ascending and may need to consider descent, depending on the severity of illness and circumstances. Individuals with AMS may resume ascending once symptoms resolve. Further ascent or reascent to a previously attained altitude should never be undertaken if there are ongoing symptoms. Descent should be initiated in any suspected HACE victim or if symptoms of AMS are worsening despite treatment with acetazolamide or dexamethasone. Dexamethasone is considered to be a more reliable pharmacological treatment for moderate to severe AMS/HACE than acetazolamide. If descent is not feasible, supplemental oxygen or a portable hyperbaric chamber should also be used.
Supplemental Oxygen: Oxygen, if available, delivered by nasal cannula or mask at flow rates sufficient to relieve symptoms provides a suitable alternative to descent. It should also be used when descent is recommended but not feasible or during descent in severely ill individuals. An SpO2 > 90% is usually adequate. Short-term oxygen use in the form of over-the-counter oxygen canisters should not be relied on for this purpose.
Portable Hyperbaric Chambers: Portable hyperbaric chambers are effective for treating severe altitude illness but require constant tending by care providers and are difficult to use with claustrophobic or vomiting patients. In many cases, ill individuals may improve enough that they can assist with their evacuation and descend once symptoms improve. Use of a portable hyperbaric chamber should not delay descent in situations where descent is required.
Acetazolamide: Acetazolamide should be considered for treatment of AMS. (Table 1).
Dexamethasone: Dexamethasone is very effective for treating AMS. The medication does not facilitate acclimatization, so further ascent should be delayed until the patient is asymptomatic while off the medication. Extensive clinical experience supports using dexamethasone in patients with HACE (Table 1).
High Altitude Pulmonary Edema
HAPE Prevention
Suggested Approach: Because the rates of acclimatization and physiologic responses to high altitude vary considerably among individuals, the ascent profile recommendations presented here do not guarantee HAPE prevention in all high altitude travelers. A gradual ascent profile is the primary method for preventing HAPE; the recommendations provided above for AMS and HACE prevention also apply to HAPE prevention. Pharmacologic prophylaxis should only be considered for individuals with a history of HAPE, especially multiple episodes.
Gradual Ascent: There is a clear relationship between rate of ascent and disease incidence. Gradual ascent is recommended to prevent HAPE.
Nifedipine: A calcium channel blocker that promotes vasodilation and lowers pulmonary artery pressure, nifedipine should be started the day prior to ascent and continued either until descent is initiated or the individual has spent 4 days at the highest elevation, perhaps up to 7 days if the individual ascended faster than recommended ascent rates (Table 1).
Tadalafil: Tadalafil can be used for HAPE prevention in known susceptible individuals who are not candidates for nifedipine (Table 1). There is no role for concurrent use of nifedipine and tadalafil.
Preacclimatization and Staged Ascent: Staged ascent and preacclimatization may offer a reasonable means of HAPE prevention. However, uncertainty remains as to the magnitude and duration of moderate altitude exposure necessary to yield benefit.
HAPE Diagnosis
The diagnosis of HAPE requires a very specific clinical context–an unacclimatized lowlander ascending to elevations ≥2500 m–and relies on a characteristic set of symptoms, including dyspnea on exertion out of proportion to previous experiences at high altitude or that experienced by other individuals at the same elevation. Nonproductive cough, fatigue, weakness, and gurgling sensation in the chest may also be present. With progression, individuals become dyspneic with mild exertion or at rest and may develop cyanosis and cough productive of pink frothy sputum. If available, pulse oximetry can confirm the presence of hypoxemia out of proportion to that expected for a given elevation, however, care must be exercised when using fingertip oximeters at high altitude, as oxygen saturation changes rapidly in response to small changes in oxygen tensions at high altitude and device accuracy declines with arterial oxygen saturations of less than 80%. The normal oxygen saturation at a given elevation may not be known with certainty and should be viewed as a range of values, rather than a specific number. For these reasons, clinical decisions should not be based on small differences in saturation over time or among individuals. Effort should also be made to minimize factors that cause measurement errors, including cold extremities, excess ambient light, and ill-fitting oximeter probes.
Consideration should be given to other causes of respiratory symptoms at high altitude, such as asthma, bronchospasm, pneumonia, pneumothorax, pulmonary embolism, viral upper respiratory tract infection, heart failure, or myocardial infarction.
HAPE Treatment
Suggested Approach: If HAPE is suspected or diagnosed, oxygen should be started, if available, and descent initiated to lower elevation. If descent is infeasible or delayed, supplemental oxygen should be continued, or the individual should be placed in a portable hyperbaric chamber. Individuals who develop HAPE may consider further ascent to higher altitude or reascent only when symptoms of HAPE have completely resolved and they maintain stable oxygenation at rest and with mild exercise while off supplemental oxygen and/or vasodilator therapy.
Descent: As with AMS and HACE, descent remains the single best treatment for HAPE. Individuals should try to descend at least 1000 m or until symptoms resolve. They should exert themselves as little as possible while descending (e.g. travel without a pack or via motor vehicle, helicopter or animal transportation) because exertion can further increase pulmonary artery pressure and exacerbate edema formation.
Supplemental Oxygen: When available, supplemental oxygen sufficient to achieve an SpO2 > 90% or relieve symptoms should be used while waiting to initiate descent, when descent is infeasible and during descent in severely ill patients.
Portable Hyperbaric Chambers: As for AMS and HACE, portable hyperbaric chambers can be used for HAPE treatment when descent is infeasible or delayed or supplemental oxygen is unavailable.
Nifedipine: Nifedipine should be used for HAPE treatment when descent is impossible or delayed and reliable access to supplemental oxygen or portable hyperbaric therapy is unavailable (Table 1).
Phosphodiesterase Inhibitors: By virtue of their ability to cause pulmonary vasodilation and decrease pulmonary artery pressure, there is a strong physiologic rationale for using phosphodiesterase inhibitors in HAPE treatment. Therefore, tadalafil or sildenafil can be used for HAPE treatment when descent is impossible or delayed, access to supplemental oxygen or portable hyperbaric therapy is impossible, and nifedipine is unavailable.
Continuous Positive Airway Pressure (CPAP): Positive airway pressure works by increasing transmural pressure across alveolar walls, thereby increasing alveolar volume and subsequent gas exchange. CPAP may be considered for treatment of HAPE when supplemental oxygen or pulmonary vasodilators are not available or as adjunctive therapy in patients not responding to supplemental oxygen alone.
Patients with Concurrent HAPE and HACE
For HAPE patients with neurologic dysfunction that does not resolve rapidly with administration of supplemental oxygen and improvement in the patient’s oxygen saturation, dexamethasone should be added to the treatment regimen at the doses described for HACE (Table 1). Nifedipine or other pulmonary vasodilators may be used in patients with concurrent HAPE and HACE, with care to avoid lowering systemic blood pressure.
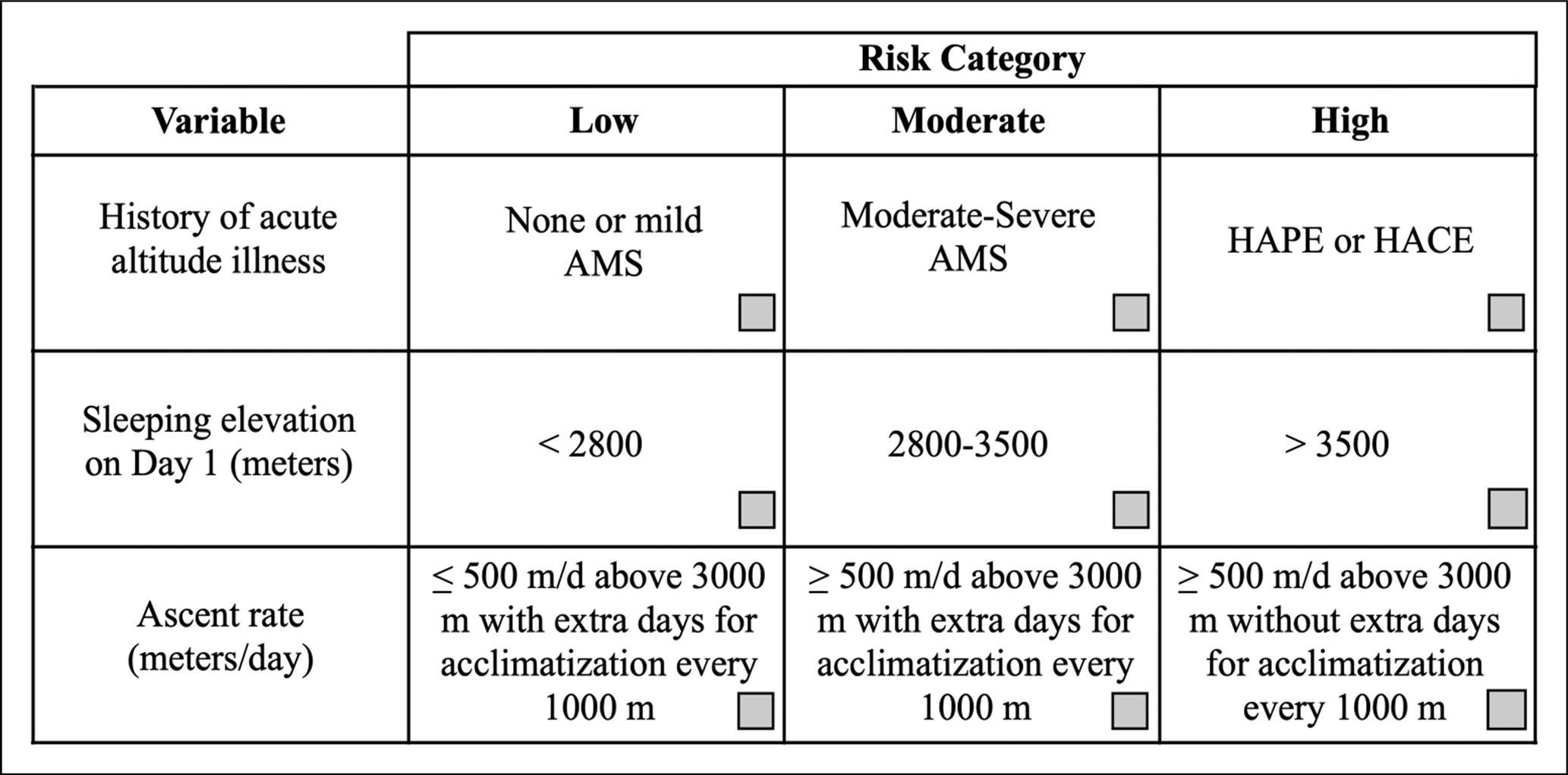
Figure 1. Assessing the risk of acute altitude illness. Medical history and features of the planned ascent can be used to assess the risk of acute altitude illness after ascent. Check marks should be placed in the boxes that best describe the variables in the left-hand column. The risk of a planned ascent is determined by the farthest column to the right in which a check mark is placed. This assessment applies to unacclimatized individuals. Ascent is assumed to start from elevations <1200 m. A history of acute altitude illness does not necessarily reflect high risk with all future ascents, as a slower ascent rate or lower target elevation on subsequent trips may help avoid problems. The risk of travel above any given elevation can be mitigated by ensuring an appropriately slow rate of ascent. The severity of prior AMS can be graded using the information in Table 2. AMS, acute mountain sickness; HACE, high altitude cerebral edema; HAPE, high altitude pulmonary edema.
Table 1. Recommended Dosages for Medications Used in the Prevention and Treatment of Altitude Illness
|
Medication
|
Indication
|
Route
|
Dosage
|
|
Acetazolamide
|
AMS, HACE Prevention
|
Oral
|
125 mg every 12 h 1, 2
Pediatrics: 1.25 mg•kg-1 every 12 h (maximum 125 mg per dose)
|
|
AMS Treatment 3
|
Oral
|
250 mg every 12 h Pediatrics: 2.5 mg•kg-1 every 12 h (maximum: 250 mg per dose)
|
|
Dexamethasone
|
AMS, HACE Prevention
|
Oral
|
2 mg every 6 h or 4 mg every 12 h 1
Pediatrics: Should not be used for prophylaxis
|
|
AMS, HACE Treatment
|
Oral, IV, IM
|
AMS: 4 mg every 6 h
HACE: 8 mg once then 4 mg every 6 h
Pediatrics: 0.15 mg•kg-1•dose-1 every 6 h (Maximum: 4 mg per dose)
|
|
Ibuprofen
|
HAH Treatment
|
Oral
|
600 mg every 8 h
|
|
Nifedipine
|
HAPE Prevention
|
Oral
|
30 mg ER version, every 12 h or 20 mg ER version every 8 h 4
|
|
HAPE Treatment
|
Oral
|
30 mg ER version, every 12 h or 20 mg ER version every 8 h
|
|
Tadalafil
|
HAPE Prevention
|
Oral
|
10 mg every 12 h 4
|
|
Sildenafil
|
HAPE Prevention
|
Oral
|
50 mg every 8 h 4
|
AMS: Acute mountain sickness; ER: extended release; HACE: High altitude cerebral edema; HAH: High altitude headache; HAPE: High altitude pulmonary edema
1 For individuals ascending to and remaining at a given elevation, following arrival at the target elevation, the medication should be continued for 2 days in individuals adhering to the recommended ascent rate and 2-4 days in individuals ascending faster than recommended rates. Individuals who ascend to a target elevation and immediately descend can stop the medication once descent is initiated.
2 This dose applies to low-moderate risk ascent profiles. For high-risk ascent profiles, consider 250 mg BID. The appropriate dose for ascent above 5000 m is not clear.
3 Acetazolamide can also be used at this dose as an adjunct to dexamethasone in HACE treatment, but dexamethasone remains the primary treatment for HACE.
4 For individuals requiring HAPE prophylaxis, ascending to and remaining at a given elevation, following arrival at the target elevation, the medication should be continued for 4 days in individuals adhering to the recommended ascent rate and 4-7 days in individuals ascending faster than recommended rates. Individuals who ascend to a target elevation and immediately descend can stop the medication once descent is initiated.
Table 2. Acute Mountain Sickness Classification
|
Category
|
Mild AMS
|
Moderate-Severe AMS
|
High Altitude Cerebral Edema (HACE)
|
|
Symptoms
|
Headache plus one or more other symptoms (nausea/vomiting; fatigue, lassitude, dizziness).
All symptoms of mild intensity
|
Headache plus one or more other symptoms (nausea/vomiting; fatigue, lassitude, dizziness).
All symptoms of moderate-severe intensity
|
Worsening of symptoms seen in moderate to severe AMS
|
|
Signs
|
None
|
None
|
Ataxia, severe lassitude,
altered mental status,
encephalopathy
|
|
Lake Louise AMS Score
|
3-5
|
6-12
|
Not applicable
|